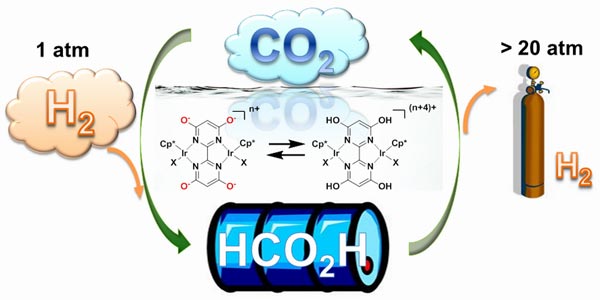
Scientists at the Brookhaven National Laboratory, with the help of its collaborators, have developed a new catalyst that can reversibly convert hydrogen gas into carbon dioxide to produce a liquid, called formic acid. Hydrogen has always remained an attractive source of fuel, especially as it does not produce any toxic products or even greenhouse gasses. But, one major deterrent that forbids its use is storage and transportation, which can be dealt with the production of this liquid form. By adjusting the pH, it can then be used in carbon neutral energy applications.
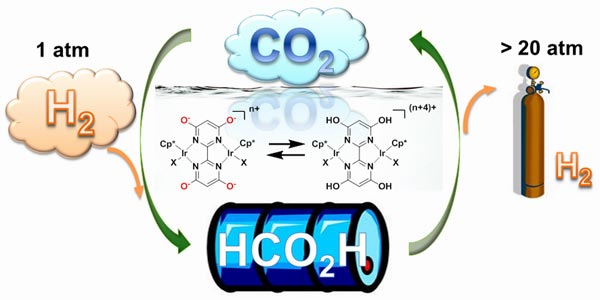
Of course, we have to agree that this is not the first effort of its kind, but what makes it different is that it will work at room temperatures, under atmospheric pressure in an aqueous solution, like water. This allows for both forward and backward reactions. The acidity determines the direction of the reaction. If you desire to release hydrogen, you simply have to flip the pH switch and the catalyst will run a backward reaction.
The purpose behind creating a new catalyst was to efficiently move protons and electrons from some molecules and then placing them of others for producing the desired liquid. If under right conditions, hydroxyl groups on the diimine ligand will allow hydrogen and carbon dioxide to react with one another.
In fact, the scientists were able to convert a mixture of hydrogen and carbon dioxide in the ratio 1:1 to make formic acid at room temperature. This is one among the efforts made to develop a usable catalyst efficiently and further work can prove rather beneficial for storing and transportation.
Via: Physorg