Eco Factor: Efficiency of lithium-air batteries to be improved using a catalyst composed of gold and platinum.
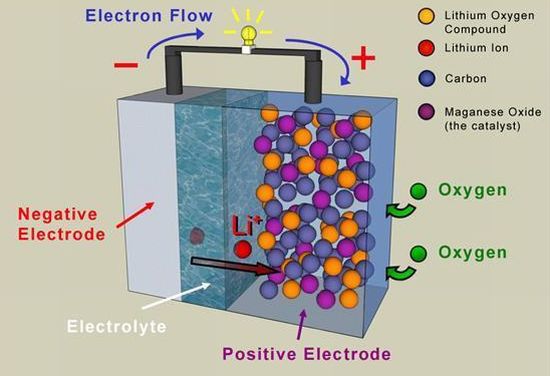
Researchers at MIT have developed a catalyst that has the potential of making rechargeable lithium-air batteries significantly more efficient. This catalyst that comprises of nanoparticles of a gold and platinum alloy could lead to returning 85 to 90 percent of the energy used to charge the battery as electricity when discharged. Gold and platinum alloy nanoparticles sit on top of a carbon black substrate, improving the efficiency of lithium-air batteries.
Lithium-air batteries, which produce electricity by reacting lithium metal and oxygen from the air, have their share of problems too. Not only do they last a few dozen charges and discharge cycles, they also release their energy slowly and are prone to contamination by carbon dioxide and water. Addressing one of their most serious problems, the new catalyst makes the battery more efficient and longer lived.
In addition to improving efficiency, promoting these reactions could also potentially increase the number of times that lithium-air batteries can be recharged, by minimizing the accumulation of lithium oxide, which otherwise clogs up the battery. The scientists are also working toward reducing the cost of the catalyst by using less platinum and gold. The MIT team sees this as a step toward making these high-energy-density batteries practical for use in electric vehicles and other areas.
Via: Technology Review