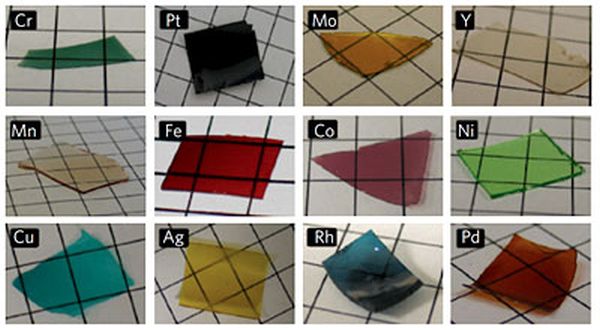
Although, it has been annoying challenge but engineers have been looking forward to build porous metal films for electrodes in batteries and catalysts in fuel cell that is highly conductive and avails more surface area for chemical reactions for transferring electricity. This year a method of making porous metal films, which provide 1000 times better electrical conductivity as compared to previous methods has been developed by Cornell chemists. With the new technique wide variety of metal nanostructures for biomedical applications and engineering can also be created.
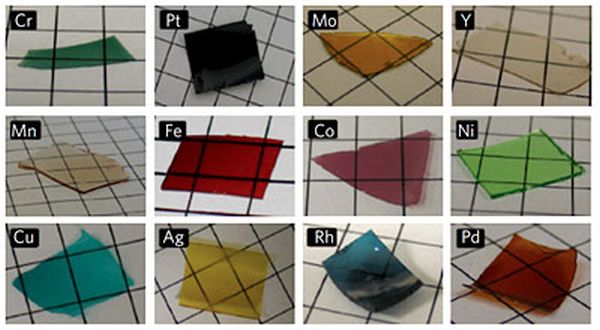
According to Ulrich Wiesner, the Spencer T. Olin Professor of Engineering, extraordinary control levels on nanostructure, functionality and composition have been reached. The base of the new method is sol gel process that is already well known to chemists. Specific silicon compounds are mixed with solvents that will assemble themselves into a silicon dioxide structure (i.e. glass) honeycombed with nanometer scaled pores. The challenge for researchers was the addition of metal in order to make the porous structure good conductor of electricity. Around ten years ago, to generate electricity, research group of Wiesner collaborated with Cornell Fuel Cell Institute also tried to use the sol gel process with the catalysts capable of pulling off protons of fuel molecules but due to addition of more than a small amount of metal the sol gel process was disturbed. They also needed materials capable of passing high current.
This time an idea to use amino acid for linking silica molecules to metal atoms came up in the mind of Warren, Ph.D student in group of Weisner then and now a researcher at Northwestern University. He had realized the two ends of amino acid molecule are attracted to metals and silica molecules respectively. The immediate outcome was nanostructure of silica, metal and carbon with more metal than ever before increasing the conductivity greatly and after removing carbon and silica all we are left with porous metal. It was noted by Warren that silica metal structure is capable of holding its shape in the high temperatures found in some fuel cells and if only silica is removed, leaving carbon metal complex, other possibilities are offered including large pores.
Via: Cornell