With fossil fuel sources depleting and global warming on the rise, exploring alternative means of power for humans is a necessary reality. Now, looking to the sky, relying on the wind or harnessing water power are not the only remaining options. Deep within the Earth is an untapped source of energy: geothermal energy. Geothermal power is expensive to tap into (though cheap to maintain) because it involves an exploration and drilling process similar to oil. As with other types of power, sky-high oil prices are making it more attractive. It’s as clean as energy comes, and a well-managed field churns up a steady stream of steam power day in and day out for decades at a time.
I’m sure you all want to know why helium isotopes found in surface waters can lead to sources of geothermal energy. Here is an explanation: Earth’s crust contains a variety of noble gases, one of those being helium. Natural helium occurs as two isotopes, helium-4 (4He) and helium-3 (3He.) Typically, helium-4 is more abundant in Earth’s crust, whereas helium-3 is more abundant in the mantle below. Thus, the helium-3/helium-4 ratio of the gas found in groundwater can provide an indication of the extent to which the water has interacted with volcanic rocks derived from the mantle.
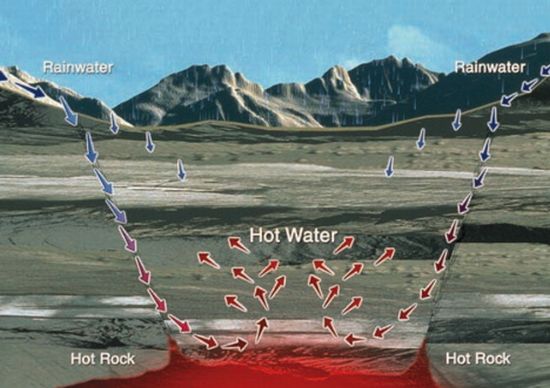
A good geothermal energy source has three basic requirements: a high thermal gradient which means accessible hot rock plus a rechargeable reservoir fluid, usually water, and finally, deep permeable pathways for the fluid to circulate through the hot rock. Geothermal energy is entirely domestic, making it more reliable than oil or natural gas. And it’s entirely renewable if managed properly, making its prices, if not lower, then at least more predictable.
Via: Treehugger