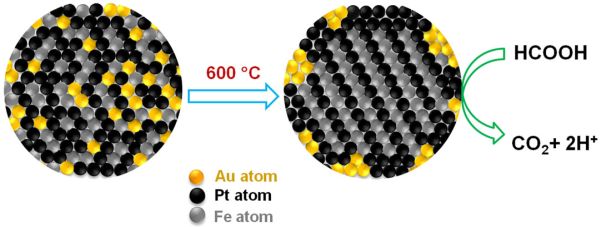
The main limiting factor that is currently slowing down the process of fuel cell development is the inadequate studies made with respect to metallic catalysts. In this regard, Chemists at Brown University experimented with gold and developed a triple headed metallic nanoparticle, which is relatively more efficient and more durable. In fact, they claim it is the addition of gold, which will yield a uniform crystal structure and at the same time, also remove carbon monoxide from the reaction.
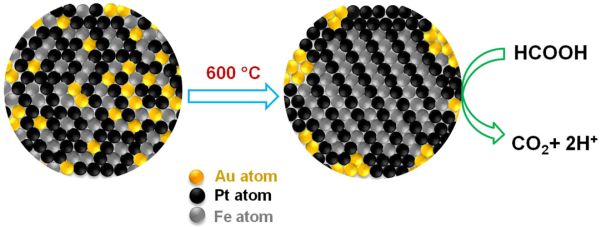
According to the study, researchers claim that 4 nanometer triple headed catalyst, which is composed of iron, platinum and gold (FePtAu), can generate higher current for every unit of mass, when compared with any other nanoparticle catalyst.
The reason why gold is the highlighted element of the trimetallic nanoparticle is that it is the key reactor. It performs the task of an organizer and leads the iron and platinum atoms into uniform layers inside the nanoparticle. Then the gold particles exit and bind with the outside surface, creating more space for the iron and platinum atoms for assembling themselves. Gold is also very useful as it catalyzes the process of oxidation and removes carbon monoxide.
As already mentioned, gold is the organizer and as part of the same it creates order for the crystal structure called “face centered tetragonal”. A four sided structure is formed and within it, the iron and platinum atoms occupy specific positions, thereby, creating order.
All experiments in place show that iron-platinum-gold (FePtAu) catalyst attained 2809.9Ma/mg Pt i.e. mass activity generated per milligram of platinum. This was found to be the highest that any nanoparticle catalyst has ever reported to generate. After 13 hours too, the mass activity of the FePtAu nanoparticle stands at, 2600Ma/mg Pt, which is 93 percent of its original performance.
This project has been funded by the U.S. Department of Energy and the Exxon Mobil Corporation and researchers will further experiment with metals other than gold for further improving the performance of the catalyst and its durability.
Via: Sciencedaily