The dwindling sources of natural resources are a deep concern for our world. The world is already in a crisis with acute shortage of energy and the situation is almost turning unmanageable with no more energy resources to tap than which are already there and are fast becoming extinct. Human beings are fast recognizing the importance of tapping the potential of renewable energy which is available in abundance and never exhausted. Here are some newly discovered forms of renewable energy for your daily needs.
1. MIT scientists successful in creating a stable artificial leaf

This path breaking discovery in the field of chemistry is made by the researchers at Nocera Lab, MIT. Dr Daniel Nocera and his team developed this artificial leaf with the help of electronic components, chemical catalysts like nickel and cobalt for the reaction to take place and silicone. The artificial leaf thus created can use sunlight for photosynthesis which is a natural plant process. The photosynthesis helps in the isolation of water into hydrogen and oxygen. Hydrogen that is thus produced can be used for the production of clean and green electricity for our daily needs. This artificial photosynthesis can turn out to be a wonderful way to sustain natural resources and restore the lost balance.
2. Botryococcus braunii for the replacement of natural fuels
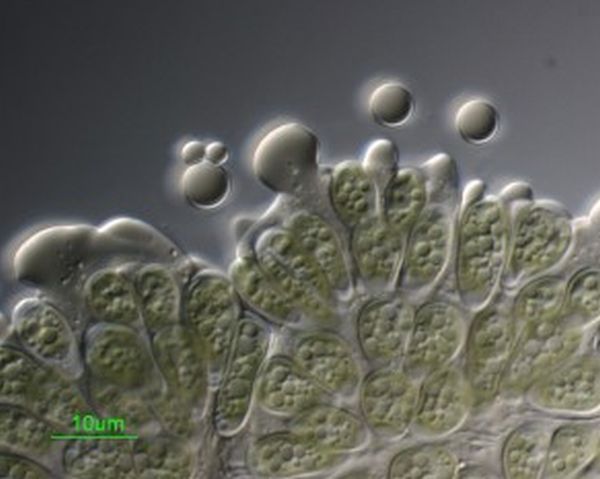
A team of researchers have identified the molecular mechanism that leads to formation of oil as well as coal shale deposits. The hydrocarbons that are responsible for the production of oil deposits have been identified in a unique form of alga, Botryococcus braunii. The alga is still found to be in existence and the researchers have successfully identified the biochemical traits of these genes and have produced genetically engineered yeast that help in the production of oil. The research which is still at its nascent stage will bring about a huge revolution as it can be used for producing a replacement for naturally occurring fuel. However, the alga is known to be very slow growing and not a great source of bio fuels. Scientists can still benefit from it if they identify the genetic blue print required for the biosynthesis of oils so that they can use it for developing alternative sources.
3. Sun’s heat for production of hydrogen

A concept being developed by researchers Sossina Haile and William Chueh, the idea revolves around using sunlight as well as water for creating energy that can be virtually used for powering anything and everything. Concentrated heat that is coming from the sun is used for converting carbon dioxide and water into carbon monoxide and hydrogen. High amounts of these gases can be used to form liquid fuel. These two fuels can also be used for producing methane for electricity generators that are fired with gas. The purity of the fuels used makes them even suitable for running fuel cells too.
For converting carbon dioxide and water to CO and H2, normally expensive elements are used. But Haile explored the possibility of using Ceria, a metal that is abundantly available and cheaper. Cerium is heated in high temperature to release oxygen and then the temperature is lowered in the presence of water and carbon dioxide to form CO and H2. This is made possible because the oxygen from water and carbon dioxide migrates to the space left by the oxygen molecules that were released when the cerium was heated in high temperature.
4. Bacteria Make Hydrogen Fuel From Water
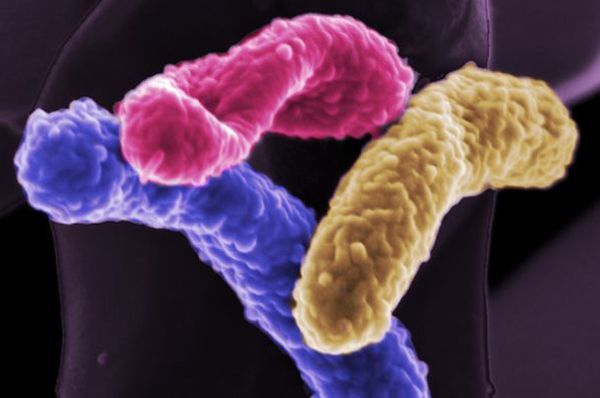
The manufacturing of hydrogen from water and carbon dioxide requires expensive catalysts and hence not an easy option in most cases. Researchers have been looking at various viable options to produce hydrogen from cheaper sources. Bacteria are one of the latest identified ways that can be used for the production of hydrogen. In year 2009, scientists discovered that there are certain unique strands of bacteria that are capable of segregating hydrogen and oxygen molecules from water. The bacteria are triggered into action with the use of electric charge which is again an expensive procedure. To combat the expense, an electro chemical reaction between salt water and freshwater has been identified.
The procedure involves the use of a membrane between salt water and freshwater so that the ions can flow freely through and the water molecules can be curtailed as well. The osmotic forces are balanced by the passage of ions to the freshwater. The charge difference thus created, even though very small, is sufficient for activating the bacterial action for producing hydrogen.




