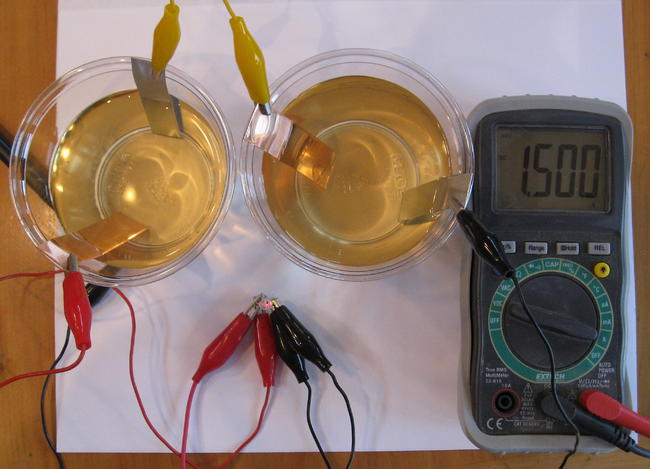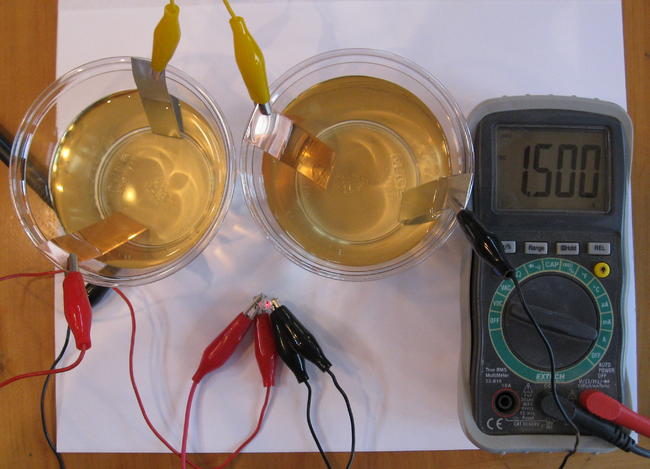
It is very important to remember that we cannot keep exploiting the non-renewable energy sources for long. We have to find substitutes to the conventional forms of energy which do not pollute our environment and are efficient as well. Here are certain examples of DIY batteries which are eco-friendly and easy to make. The cells we are going to discuss are supposed to be connected in a series to receive an effective output.
1. Battery using fruits
Acidic fruits are tasty and capable of producing electricity which can be harnessed easily. These fruits are eco-friendly, cheap and reusable. Things required to construct this kind of a battery are – zinc bar, copper bar and fruits. Insert copper and zinc bars into the fruit in such a way that they do not touch each other. Many cells of this kind can be connected together to make an effective battery. Connect positive of one fruit to negative of another fruit. Same type of fruit should not be attached consecutively.
2. Battery using salt water or vinegar
We take two dissimilar metals like copper and iron rod and insert them into an electrolyte that is salt water or vinegar. The dry ends of the terminal will give some current. This is a cell, to make a battery you have to join more number of cells.
3. Battery using mud
We take an empty container and pour blackish brown mud into it. One of the copper wires is attached to the aluminum foil while the other is attached to the graphite rod. Now the two wires act as anode and cathode of the cell which is immersed in the mud. These cells are connected in series to make an effective battery. These kinds of batteries are also known as Earth Battery.
4. Battery using rain water
We need a PVC pipe, a zinc pipe, a copper pipe and rain water for the construction of the rain water battery. Cut the PVC pipe in two parts and attach it with the T-connector. Drill some holes in the copper pipe at equal intervals for the water to get in. Now put the copper pipe into the PVC pipe and then the zinc pipe into the copper pipe in a way that their extreme ends are out of the PVC pipe. Seal the ends of the PVC pipe to make it water proof. Attach the pipe to the container of rain water through the T-connector. This kind of battery gives 1 volt of current.
5. Battery using bacon
Here we require few pieces of bacon, copper and zinc plated wires. Copper and zinc act as cathode and anode of the battery while the bacon act as the medium (electrolyte). A group of copper and zinc wires are attached together and inserted in the bacon. Each slice of bacon comprises of one copper electrode and one zinc electrode. The thicker the slice of bacon better is its conductivity. A single bacon cell provides us with 0.6 volts but when grouped with more than 7 or 8 gives an optimum amount of energy.
6. Battery using lemon
We require some ferrite bead, copper wire, sheets of zinc, copper and lemon. The lemon has to be pressed with a little force to reduce its internal resistance till it becomes soft and juicy. Then we make some vertical cuts on it. Now, electrodes are imbedded in the lemon in a sequence where copper is placed before zinc and the arrangement is repeated again and again, making sure the rods do not touch each other. Join the joule thief to the lemon. Finally the twisted transformer is attached to the copper and the negative leg is connected to zinc rod respectively.
7. Battery using soda
A plastic glass, copper & zinc strip, a knife and soda are the things required for the construction of soda battery. Two holes are made at the bottom of a glass. We insert the two electrodes from the holes and then pour soda into the glass. To increase the output make the second apparatus of the same type and attach zinc rod of the first apparatus to the copper rod of the second. The voltage increases at even intervals of time.
8. Battery using bleach
We need two different metals like aluminum bar and copper pipe. We insert these metals into a glass filled with Clorox bleach. Now wires are drawn from both the dry ends of the two metals to obtain some voltage. You receive more current when you add more bleach to the solution, but at the same time the metal corrodes at a faster rate. Hence you should add less bleach to prevent the corrosion and in order to get high voltage you should attach more number of cells together.
9. Battery using zinc
Make a mixture of 50 ml sodium hydroxide with 150 ml of water. When both are mixed heat is liberated, so the mixture should be carefully kept aside to cool down. One end of the wire is attached to the zinc sheet and the other is attached to the steel wool. The latter is then wrapped with the paper towel to act as a separator. Zinc is wrapped around the steel wool and the whole apparatus is placed into a container. Steel wool should be exposed to air to get in contact with the oxygen. Now, we pour the sodium hydroxide solution into the container to complete the circuit.
10. Battery using copper sulphate
We take a solution of copper sulphate and drop an excess of zinc metal into it. Let it stay overnight until the solution clears, leaving some residue of copper metal at the bottom of the container. Then we pour the solution of zinc sulphate into a fresh beaker. One end of the copper wire is put into the bottom of the solution and the other end is pushed out of the beaker. Now, we suspend a zinc plate above the copper wire. A 0.95 voltage is observed.