 Making about 75 percent of all matter, hydrogen is the most abundant element in the universe. This vast presence makes scientists and researchers think about hydrogen as the clean fuel of the future. However, hydrogen fuel does not occur naturally on Earth and thus is not an energy source, but is an energy carrier. Currently it is most frequently made from methane or other fossil fuels, which makes hydrogen generation not as clean as the end product. The answers lie in generating hydrogen from water using electrolysis in the presence of one or more catalysts. Check out 10 such ways that can be used to produce clean hydrogen by electrolyzing water in an energy-efficient way: • Hydrogen from water using sunlight:
Making about 75 percent of all matter, hydrogen is the most abundant element in the universe. This vast presence makes scientists and researchers think about hydrogen as the clean fuel of the future. However, hydrogen fuel does not occur naturally on Earth and thus is not an energy source, but is an energy carrier. Currently it is most frequently made from methane or other fossil fuels, which makes hydrogen generation not as clean as the end product. The answers lie in generating hydrogen from water using electrolysis in the presence of one or more catalysts. Check out 10 such ways that can be used to produce clean hydrogen by electrolyzing water in an energy-efficient way: • Hydrogen from water using sunlight: 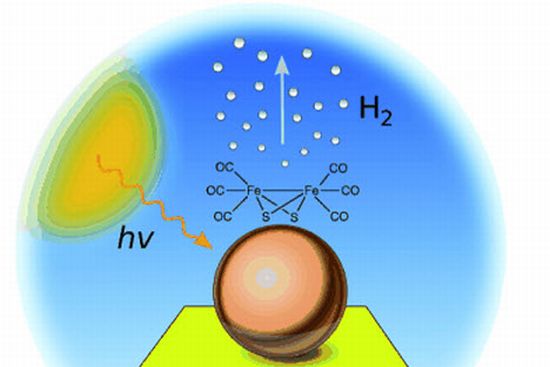 Researchers at the University of East Anglia have reported a breakthrough in hydrogen generation from water, with a new system that achieves 60 percent efficiency for a process in which hydrogen is produced from water by the photons striking a specially designed electrode. The technology is based on the use of a nanophotocathode, which is a gold electrode coated with nanoclusters of indium phosphide. • Using virus to generate hydrogen from water:
Researchers at the University of East Anglia have reported a breakthrough in hydrogen generation from water, with a new system that achieves 60 percent efficiency for a process in which hydrogen is produced from water by the photons striking a specially designed electrode. The technology is based on the use of a nanophotocathode, which is a gold electrode coated with nanoclusters of indium phosphide. • Using virus to generate hydrogen from water:  A team of researchers at MIT have developed a process to replicate the natural process of photosynthesis in plants by engineering the M13 bacterial virus that can split water into its constituent molecules that is hydrogen and oxygen using abundant solar energy. The team hopes that the process can further be used to create hydrogen efficiently from water, which can later be used to produce electricity. • Using homogenous catalyst from water oxidation:
A team of researchers at MIT have developed a process to replicate the natural process of photosynthesis in plants by engineering the M13 bacterial virus that can split water into its constituent molecules that is hydrogen and oxygen using abundant solar energy. The team hopes that the process can further be used to create hydrogen efficiently from water, which can later be used to produce electricity. • Using homogenous catalyst from water oxidation:  Chemists at Emory University have developed the most potent homogeneous catalyst known for water oxidation, which is being considered a crucial component for generating hydrogen fuel from water using only sunlight. The research aims to mimic natural processes such as photosynthesis to generate clean fuel, with the long-term goal to generate hydrogen from water using only sunlight. • Using water photo-oxidizing catalyst:
Chemists at Emory University have developed the most potent homogeneous catalyst known for water oxidation, which is being considered a crucial component for generating hydrogen fuel from water using only sunlight. The research aims to mimic natural processes such as photosynthesis to generate clean fuel, with the long-term goal to generate hydrogen from water using only sunlight. • Using water photo-oxidizing catalyst: 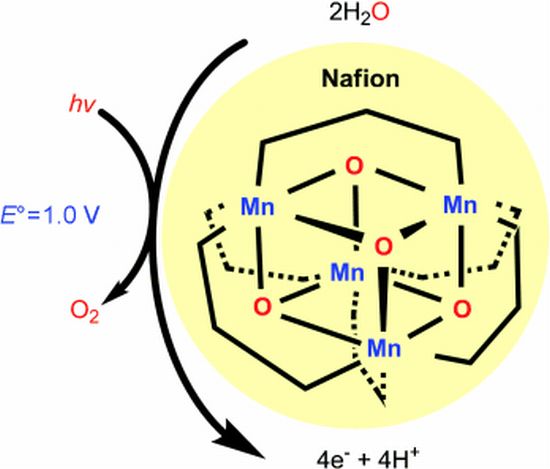 An Australian-US research team led by Monash University has developed a bio-inspired water photo-oxidizing catalyst for the splitting of water into oxygen and hydrogen using solar energy. This allows us to produce loads of hydrogen by splitting water and while the process has always been something on the horizon for clean energy, this will increase the efficiency of it all. The researchers have mimicked nature, taking the elements and mechanisms found in plant life that have evolved over 3 billion years and recreated one of those processes in the laboratory. • Using Iridium oxide molecules:
An Australian-US research team led by Monash University has developed a bio-inspired water photo-oxidizing catalyst for the splitting of water into oxygen and hydrogen using solar energy. This allows us to produce loads of hydrogen by splitting water and while the process has always been something on the horizon for clean energy, this will increase the efficiency of it all. The researchers have mimicked nature, taking the elements and mechanisms found in plant life that have evolved over 3 billion years and recreated one of those processes in the laboratory. • Using Iridium oxide molecules:  Researchers at Pennsylvania State University have developed a catalyst that consists of iridium oxide molecules surrounded by orange-red dye molecules. They chose the orange-red dye as it had the capacity to absorb sunlight in the blue range that has maximum energy. The catalyst was then impregnated to a titanium dioxide electrode to act as the anode and a platinum electrode served as the cathode. The electrodes were then immersed in a salt solution and were separated from each other to avoid the component gases in the solution from recombining. Sunlight falling on the dye-sensitive anode would start the process of splitting water into its component gases. • Using molecular molybdenum-oxo catalyst:
Researchers at Pennsylvania State University have developed a catalyst that consists of iridium oxide molecules surrounded by orange-red dye molecules. They chose the orange-red dye as it had the capacity to absorb sunlight in the blue range that has maximum energy. The catalyst was then impregnated to a titanium dioxide electrode to act as the anode and a platinum electrode served as the cathode. The electrodes were then immersed in a salt solution and were separated from each other to avoid the component gases in the solution from recombining. Sunlight falling on the dye-sensitive anode would start the process of splitting water into its component gases. • Using molecular molybdenum-oxo catalyst:  Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory and the University of California, Berkeley have developed a new catalyst that can replace the use of expensive platinum to generate hydrogen from water. The new catalyst, which is a molecular molybdenum-oxo catalyst, doesn’t require any organic additives and can operate in neutral water, even if it’s dirty. As an added advantage, the catalyst is seventy times cheaper than platinum. • Using waste energy:
Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory and the University of California, Berkeley have developed a new catalyst that can replace the use of expensive platinum to generate hydrogen from water. The new catalyst, which is a molecular molybdenum-oxo catalyst, doesn’t require any organic additives and can operate in neutral water, even if it’s dirty. As an added advantage, the catalyst is seventy times cheaper than platinum. • Using waste energy:  Scientists at the University of Wisconsin-Madison have developed a novel way to generate hydrogen by breaking bonds of water using waste energy in the form of noise and stray vibrations. The scientists grew nanocrystals of two common crystals zinc oxide and barium titanate and placed them in water. When pulsed with ultrasonic vibrations, the nanofibers flexed and catalyzed a chemical reaction to split a water molecule into its constituents. The fibers can be tuned to convert waste energy in the form of noise and vibrations into electrical energy, which rather than being harvested directly is used to break the bonds of water and generate oxygen and hydrogen. • Solar-powered hydrogen plant uses sea water:
Scientists at the University of Wisconsin-Madison have developed a novel way to generate hydrogen by breaking bonds of water using waste energy in the form of noise and stray vibrations. The scientists grew nanocrystals of two common crystals zinc oxide and barium titanate and placed them in water. When pulsed with ultrasonic vibrations, the nanofibers flexed and catalyzed a chemical reaction to split a water molecule into its constituents. The fibers can be tuned to convert waste energy in the form of noise and vibrations into electrical energy, which rather than being harvested directly is used to break the bonds of water and generate oxygen and hydrogen. • Solar-powered hydrogen plant uses sea water:  Conceived by SolarLab Research and Design, this Hydrogen Powerplant can be one of the safest and the most efficient ways to produce hydrogen. The offshore power plant is designed to make use of seawater, a fuel cell and solar tiles to produce hydrogen that is then pumped safely to inflated hydrogen tanks fixed on the sea floor. The water-cooling effect reduces operating temperature and increases PV efficiency by up to 30%. • Using Sun Catalytix’s electrolyzer:
Conceived by SolarLab Research and Design, this Hydrogen Powerplant can be one of the safest and the most efficient ways to produce hydrogen. The offshore power plant is designed to make use of seawater, a fuel cell and solar tiles to produce hydrogen that is then pumped safely to inflated hydrogen tanks fixed on the sea floor. The water-cooling effect reduces operating temperature and increases PV efficiency by up to 30%. • Using Sun Catalytix’s electrolyzer: 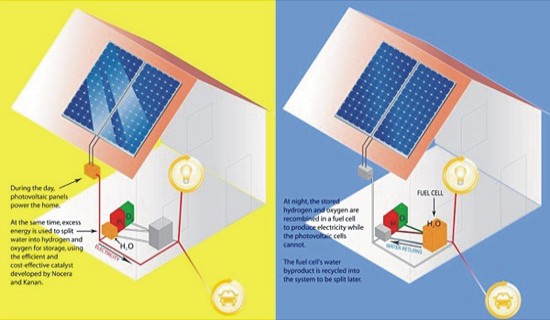 Sun Catalytix is working on a prototype that will use sun’s energy to split water into its constituent molecules that is hydrogen and oxygen using electrolysis. The company has been testing a new electrolyzer that is built around a special catalyst and can be powered by solar energy. The main idea is to use solar energy to power the electrolyzer and store the hydrogen generated in a tank. When the need for electricity arises, the hydrogen can be put through a fuel cell. The company calculates that about 3-liters of water can power an average home, or a fuel cell car. • Using titania electrode:
Sun Catalytix is working on a prototype that will use sun’s energy to split water into its constituent molecules that is hydrogen and oxygen using electrolysis. The company has been testing a new electrolyzer that is built around a special catalyst and can be powered by solar energy. The main idea is to use solar energy to power the electrolyzer and store the hydrogen generated in a tank. When the need for electricity arises, the hydrogen can be put through a fuel cell. The company calculates that about 3-liters of water can power an average home, or a fuel cell car. • Using titania electrode: 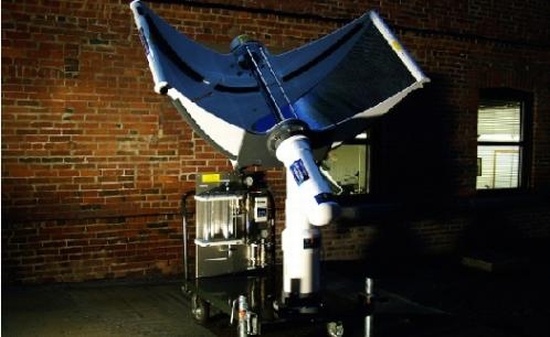 The Nanoptek Hydrogen Generator requires solar energy and water to produce hydrogen. The company developing the product claims to have come up with a titania electrode that is capable of splitting water into its constituents.
The Nanoptek Hydrogen Generator requires solar energy and water to produce hydrogen. The company developing the product claims to have come up with a titania electrode that is capable of splitting water into its constituents.
10 eco friendly ways to generate hydrogen from water




